GCP

ICH GCP E6 (R3) training for sponsors, investigators, regulators and others in 14 languages
Our GCP Investigator Site Training meets the Minimum Criteria for ICH GCP Investigator Site Personnel Training identified by TransCelerate BioPharma as necessary to enable mutual recognition of GCP training among trial sponsors.
ICH Good Clinical Practice (GCP) is the widely used international ethical, scientific and practical standard to which all clinical research is conducted. Compliance with GCP provides public assurance that the rights, safety and wellbeing of human research participants are protected and that research data are reliable.
Everyone involved in the conduct of clinical research must be competent to perform their tasks, qualified by education, training and experience.
Our training courses address the GCP aspects of this requirement.
We are currently in the process of updating our courses to be ICH GCP (E6(R3) compliant. Please check with us to ensure we fulfil your requirements.
Contact us for fantastic group rates for 5 or more participants on any course
Our online brochure can be found at https://brookwood-global.com/Books/Online-Training-Brochure/
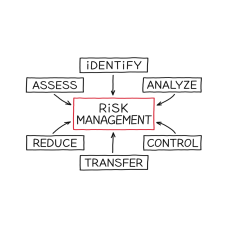
Risk Management in Clinical Trials
Risk Management in Clinical Trials In this 60-min course, we look at the general principles relat..
£100.00
A Guide to the UK Clinical Trial Regulations 2026
Available March 2026.This course overviews key UK Clinical trial regulations as implemented in April..
£100.00
The UK Clinical Trial Regulations are changing
The UK Clinical Trial Regulations are Changing This is a short update course (ca. 40 mins) that ..
£60.00
Essential GCP for Investigators - Brazilian Portuguese
Essential GCP for Investigators Online Training with Brazilian Portuguese narration ..
£100.00
Essential GCP for Investigators - Chinese
Online GCP Training Chinese with Mandarin Narration ..
£100.00
Essential GCP for Investigators - English
Essential GCP for Investigators Comprehensive training on investigator ICH GCP E6(R3) responsi..
£100.00
Essential GCP for Investigators - French
Online Good Clinical Practice with French narration ..
£100.00
Essential GCP for Investigators - Hungarian
Online GCP Training in Hungarian (read-only) In order to get your CDP ..
£100.00