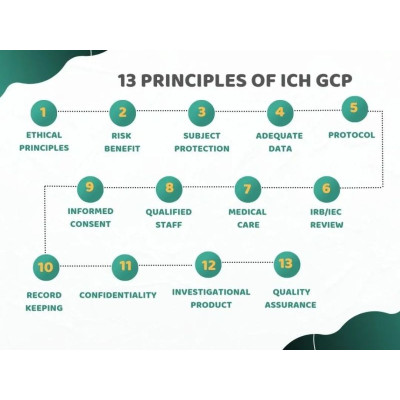
This short (30 mins) course covers the overarching
principles of GCP that are the key requirements to be followed in all clinical
trials. The principles presented in this course are reproduced from the Step 4
ICH E6(R3) guideline of January 2025.
This course is important for everyone involved in
human clinical trials whether commercial or non-commercial. It should be taken
in conjunction with the other GCP courses we offer.
It is especially suitable for those who do not need
to know the detail of their practical implementation presented in Annex 1 and
the Appendices of the E6(R3) guideline, such as administrators, coordinators
and those in departments outside clinical operations.
The course is personalised, interactive and has
narrated and read-only options.
What are the benefits of this sponsor training?
• Online, on demand. Do it anywhere! All that is needed is a strong continuous internet signal
• Globally log in at any time 24/7, 365 days a year
• Delivery by html 5 allows PC, laptop, iPad, iPhone and android mobile devices to be used
• Tracking of user activity, progress reporting by user and group
• Certification for satisfactory completion
• The training is personalized using the participant’s first name
• The training is challenging and long enough to be a true training and evaluation option
• Costs depend on the number of participants with excellent packages for multiple users
• Fix costs with a 2-year agreement or longer
• Help in the event of problems
• Valuable e-resources in the course include a Guide to Consent, an Essential GCP book and a copy of the ICH GCP E6(R3) guidelines
For details of packages for training larger groups contact us at info@brookwood-global.com
The Principles of GCP
- Product Code: The Principles of GCP
- Availability: In Stock
-
£100.00
Available Options
Related Products
e-book Principles of GCP
This e-book covers the overarching principles of GCP that are the key requirements to be followed i..
£10.00